Browse
Assessing Learning
Posted on: #iteachmsu


Posted by
almost 6 years ago
find me
Assessing Learning
Posted on: #iteachmsu


Posted by
almost 6 years ago
workout
Assessing Learning
Posted on: #iteachmsu

ASSESSING LEARNING
What I Wish I Knew Before Starting Grad School
Vanessa R. Corcoran writes about how to prepare your whole self - academic, but also emotional, physical, and relational - for graduate school. She emphasizes planning your support systems for multiple aspects of your life and not being ashamed to ask for help or more support.
Test 1
Test 2
Test 3
Test 1
Test 2
Test 3
Posted by:
Scarlet Ethan Edien
Posted on: #iteachmsu

What I Wish I Knew Before Starting Grad School
Vanessa R. Corcoran writes about how to prepare your whole self - a...
Posted by:
ASSESSING LEARNING
Tuesday, Sep 3, 2019
Posted on: #iteachmsu

ASSESSING LEARNING
Chemical testing overview
Overview
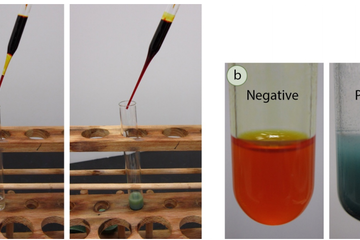
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color
Authored by:
Chathuri

Posted on: #iteachmsu


Chemical testing overview
Overview
Before spectroscopic analysis (IR, NMR) becam...
Before spectroscopic analysis (IR, NMR) becam...
Authored by:
ASSESSING LEARNING
Monday, Aug 26, 2019
Posted on: #iteachmsu
ASSESSING LEARNING
Chemical testing overview
Overview
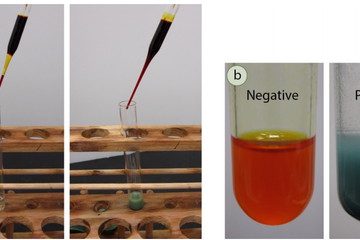
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color
Authored by:
Chathuri

Posted on: #iteachmsu


Chemical testing overview
Overview
Before spectroscopic analysis (IR, NMR) becam...
Before spectroscopic analysis (IR, NMR) becam...
Authored by:
ASSESSING LEARNING
Monday, Aug 26, 2019
Posted on: #iteachmsu
ASSESSING LEARNING
Chemical testing overview
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color
Authored by:
Chathuri

Posted on: #iteachmsu


Chemical testing overview
Before spectroscopic analysis (IR, NMR) became commonplace in the o...
Authored by:
ASSESSING LEARNING
Monday, Aug 26, 2019
Posted on: #iteachmsu


Posted by
about 6 years ago
New post
Assessing Learning
Posted on: #iteachmsu

Assessing Learning
Biodiversity
Posted by:
Rohit Shinde


