We found 51 results that contain "ewa"
Posted on: #iteachmsu


Natural resources gifted by god -- Edited
Natural resources are the raw materials and sources of energy that we use. Petrol, metals, soil, sand, wind, water, and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Natural resources are the raw materials and sources of energy that we use.
Petrol, metals, soil, sand, wind, water and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Think about the relationship between natural resources and manufactured products. In essence, we call them “natural” resources because they are things human society uses that are created (or were created in the case of fossil fuels) without human intervention.
Perpetually Renewable Resources
Perpetually renewable resources are the easiest resources to understand; these are natural resources that are constantly replenished by the Sun’s and Earth’s natural processes. For example, every day the sun delivers an average of 198 Watts of energy to every square meter (m
) of the Earth’s surface. For comparison a standard incandescent light bulb in a bedside lamp uses 40 Watts, or a 100kg person climbing a step in 2 seconds uses roughly 200 Watts. Every day without fail for the last 5 billion years (plus or minus a few hundred million years) the Sun has delivered this solar energy.
Together with geothermal energy (heat from the Earth’s interior), the Sun’s perpetual energy powers the winds, ocean currents, precipitation and most of the Earth’s plant life. Solar and geothermal natural resources currently energise a significant and growing percentage of many nations’ electrical grids. It is perpetually renewable in the sense that no matter how much we use in terms of human time-scales (e.g decades to millennia), the Sun and the Earth will always make more.
Intermediate Renewable Resources
Intermediate renewable resources are only renewable resources if we don’t use them too quickly. They are resources such as freshwater, soil, crops and trees for timber. If we didn’t use them, they would be perpetually renewable, but because they require time (on human time-scales) to regenerate or grow, we can overuse them until they are no longer available.
Freshwater is a great example of an intermediate renewable resource. Through the water cycle, the sun evaporates water from the surface of saltwater oceans that travels over land and falls back to earth as freshwater rain. This rain fills the lakes, rivers and aquifers we use for agriculture, industry and drinking water. If we use this freshwater at the same rate as the rain recharging it, then we won’t run out. If we use the freshwater faster than it recharges, then we will. Intermediate renewable resources must be carefully managed to ensure they are not depleted.
Non-renewable Resources
The last category of natural resources are the non-renewables. These are resources that will not regenerate on human time-scales. Once they have been depleted they will no longer be available and no more will be made. The most common examples of non-renewable resources are fossil fuels, so-called because most were created by processes that take millions of years. Fossil fuels include crude oil, natural gas, coal and uranium. Other non-renewable resources include metals, lithium and rare-Earth elements (REE’s), but it’s important to remember that while we may eventually run out of mineable metals and REE’s, with careful waste management, these can be recovered through recycling. However, it is not the same for fossil fuels as using them for energy alters their chemistry so they are no longer useful.
Natural resources are the raw materials and sources of energy that we use.
Petrol, metals, soil, sand, wind, water and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Think about the relationship between natural resources and manufactured products. In essence, we call them “natural” resources because they are things human society uses that are created (or were created in the case of fossil fuels) without human intervention.
Perpetually Renewable Resources
Perpetually renewable resources are the easiest resources to understand; these are natural resources that are constantly replenished by the Sun’s and Earth’s natural processes. For example, every day the sun delivers an average of 198 Watts of energy to every square meter (m
) of the Earth’s surface. For comparison a standard incandescent light bulb in a bedside lamp uses 40 Watts, or a 100kg person climbing a step in 2 seconds uses roughly 200 Watts. Every day without fail for the last 5 billion years (plus or minus a few hundred million years) the Sun has delivered this solar energy.
Together with geothermal energy (heat from the Earth’s interior), the Sun’s perpetual energy powers the winds, ocean currents, precipitation and most of the Earth’s plant life. Solar and geothermal natural resources currently energise a significant and growing percentage of many nations’ electrical grids. It is perpetually renewable in the sense that no matter how much we use in terms of human time-scales (e.g decades to millennia), the Sun and the Earth will always make more.
Intermediate Renewable Resources
Intermediate renewable resources are only renewable resources if we don’t use them too quickly. They are resources such as freshwater, soil, crops and trees for timber. If we didn’t use them, they would be perpetually renewable, but because they require time (on human time-scales) to regenerate or grow, we can overuse them until they are no longer available.
Freshwater is a great example of an intermediate renewable resource. Through the water cycle, the sun evaporates water from the surface of saltwater oceans that travels over land and falls back to earth as freshwater rain. This rain fills the lakes, rivers and aquifers we use for agriculture, industry and drinking water. If we use this freshwater at the same rate as the rain recharging it, then we won’t run out. If we use the freshwater faster than it recharges, then we will. Intermediate renewable resources must be carefully managed to ensure they are not depleted.
Non-renewable Resources
The last category of natural resources are the non-renewables. These are resources that will not regenerate on human time-scales. Once they have been depleted they will no longer be available and no more will be made. The most common examples of non-renewable resources are fossil fuels, so-called because most were created by processes that take millions of years. Fossil fuels include crude oil, natural gas, coal and uranium. Other non-renewable resources include metals, lithium and rare-Earth elements (REE’s), but it’s important to remember that while we may eventually run out of mineable metals and REE’s, with careful waste management, these can be recovered through recycling. However, it is not the same for fossil fuels as using them for energy alters their chemistry so they are no longer useful.
DISCIPLINARY CONTENT
Posted on: #iteachmsu


New life started
Natural resources are the raw materials and sources of energy that we use. Petrol, metals, soil, sand, wind, water, and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Natural resources are the raw materials and sources of energy that we use.
Petrol, metals, soil, sand, wind, water and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Think about the relationship between natural resources and manufactured products. In essence, we call them “natural” resources because they are things human society uses that are created (or were created in the case of fossil fuels) without human intervention.
Perpetually Renewable Resources
Perpetually renewable resources are the easiest resources to understand; these are natural resources that are constantly replenished by the Sun’s and Earth’s natural processes. For example, every day the sun delivers an average of 198 Watts of energy to every square meter (m
) of the Earth’s surface. For comparison a standard incandescent light bulb in a bedside lamp uses 40 Watts, or a 100kg person climbing a step in 2 seconds uses roughly 200 Watts. Every day without fail for the last 5 billion years (plus or minus a few hundred million years) the Sun has delivered this solar energy.
Together with geothermal energy (heat from the Earth’s interior), the Sun’s perpetual energy powers the winds, ocean currents, precipitation and most of the Earth’s plant life. Solar and geothermal natural resources currently energise a significant and growing percentage of many nations’ electrical grids. It is perpetually renewable in the sense that no matter how much we use in terms of human time-scales (e.g decades to millennia), the Sun and the Earth will always make more.
Intermediate Renewable Resources
Intermediate renewable resources are only renewable resources if we don’t use them too quickly. They are resources such as freshwater, soil, crops and trees for timber. If we didn’t use them, they would be perpetually renewable, but because they require time (on human time-scales) to regenerate or grow, we can overuse them until they are no longer available.
Freshwater is a great example of an intermediate renewable resource. Through the water cycle, the sun evaporates water from the surface of saltwater oceans that travels over land and falls back to earth as freshwater rain. This rain fills the lakes, rivers and aquifers we use for agriculture, industry and drinking water. If we use this freshwater at the same rate as the rain recharging it, then we won’t run out. If we use the freshwater faster than it recharges, then we will. Intermediate renewable resources must be carefully managed to ensure they are not depleted.
Non-renewable Resources
The last category of natural resources are the non-renewables. These are resources that will not regenerate on human time-scales. Once they have been depleted they will no longer be available and no more will be made. The most common examples of non-renewable resources are fossil fuels, so-called because most were created by processes that take millions of years. Fossil fuels include crude oil, natural gas, coal and uranium. Other non-renewable resources include metals, lithium and rare-Earth elements (REE’s), but it’s important to remember that while we may eventually run out of mineable metals and REE’s, with careful waste management, these can be recovered through recycling. However, it is not the same for fossil fuels as using them for energy alters their chemistry so they are no longer useful.
Natural resources are the raw materials and sources of energy that we use.
Petrol, metals, soil, sand, wind, water and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Think about the relationship between natural resources and manufactured products. In essence, we call them “natural” resources because they are things human society uses that are created (or were created in the case of fossil fuels) without human intervention.
Perpetually Renewable Resources
Perpetually renewable resources are the easiest resources to understand; these are natural resources that are constantly replenished by the Sun’s and Earth’s natural processes. For example, every day the sun delivers an average of 198 Watts of energy to every square meter (m
) of the Earth’s surface. For comparison a standard incandescent light bulb in a bedside lamp uses 40 Watts, or a 100kg person climbing a step in 2 seconds uses roughly 200 Watts. Every day without fail for the last 5 billion years (plus or minus a few hundred million years) the Sun has delivered this solar energy.
Together with geothermal energy (heat from the Earth’s interior), the Sun’s perpetual energy powers the winds, ocean currents, precipitation and most of the Earth’s plant life. Solar and geothermal natural resources currently energise a significant and growing percentage of many nations’ electrical grids. It is perpetually renewable in the sense that no matter how much we use in terms of human time-scales (e.g decades to millennia), the Sun and the Earth will always make more.
Intermediate Renewable Resources
Intermediate renewable resources are only renewable resources if we don’t use them too quickly. They are resources such as freshwater, soil, crops and trees for timber. If we didn’t use them, they would be perpetually renewable, but because they require time (on human time-scales) to regenerate or grow, we can overuse them until they are no longer available.
Freshwater is a great example of an intermediate renewable resource. Through the water cycle, the sun evaporates water from the surface of saltwater oceans that travels over land and falls back to earth as freshwater rain. This rain fills the lakes, rivers and aquifers we use for agriculture, industry and drinking water. If we use this freshwater at the same rate as the rain recharging it, then we won’t run out. If we use the freshwater faster than it recharges, then we will. Intermediate renewable resources must be carefully managed to ensure they are not depleted.
Non-renewable Resources
The last category of natural resources are the non-renewables. These are resources that will not regenerate on human time-scales. Once they have been depleted they will no longer be available and no more will be made. The most common examples of non-renewable resources are fossil fuels, so-called because most were created by processes that take millions of years. Fossil fuels include crude oil, natural gas, coal and uranium. Other non-renewable resources include metals, lithium and rare-Earth elements (REE’s), but it’s important to remember that while we may eventually run out of mineable metals and REE’s, with careful waste management, these can be recovered through recycling. However, it is not the same for fossil fuels as using them for energy alters their chemistry so they are no longer useful.
DISCIPLINARY CONTENT
Posted on: Agile testers group 2


WHAT IS WORKPLACE FLEXIBILITY?
WHAT IS WORKPLACE FLEXIBILITY?
"Flexibility is about an employee and an employer making changes to when, where and how a person will work to better meet individual and business needs. Flexibility enables both individual and business needs to be met through making changes to the time (when), location (where) and manner (how) in which an employee works. Flexibility should be mutually beneficial to both the employer and employee and result in superior outcomes." (1)
Formal flexibility policies are "officially approved human resources policies, as well as any official policies that give supervisors discretion to provide flexibility."
Informal flexibility refers to "policies that are not official and not written down but are still available to some employees, even on a discretionary basis."
While most formal work arrangements can usually be identified, organizations acknowledge that utilization statistics probably underestimate the true reach and impact of flexibility, as they cannot accurately determine the extent of informal flexibility—for example, employees who occasionally alter their work hours or work from home. (24)
CASE EXAMPLES
JP Morgan Chase found that 95% of employees working in an environment where the manager is sensitive to work and personal life—including informal flexibility— feel motivated to exceed expectations, compared to 80% of employees in environments where the manager is not sensitive to needs for informal flexibility. (24)
"At Bristol-Myers Squibb, 14% of employees have a formal flexible work arrangement. Of the remaining, 67% say they have informal flexibility. When asked about the importance of informal flexibility in terms of their intention to continue working at the company, the response is resounding: 71% say that it is 'very important.' Again, women place even greater importance on informal flexibility; 78% of women say it is 'very important' to their staying, compared to 65% of men. The retention effect is especially strong for women in management: 84% say informal flexibility helps keep them at the company." (24)
"Flexibility is about an employee and an employer making changes to when, where and how a person will work to better meet individual and business needs. Flexibility enables both individual and business needs to be met through making changes to the time (when), location (where) and manner (how) in which an employee works. Flexibility should be mutually beneficial to both the employer and employee and result in superior outcomes." (1)
Formal flexibility policies are "officially approved human resources policies, as well as any official policies that give supervisors discretion to provide flexibility."
Informal flexibility refers to "policies that are not official and not written down but are still available to some employees, even on a discretionary basis."
While most formal work arrangements can usually be identified, organizations acknowledge that utilization statistics probably underestimate the true reach and impact of flexibility, as they cannot accurately determine the extent of informal flexibility—for example, employees who occasionally alter their work hours or work from home. (24)
CASE EXAMPLES
JP Morgan Chase found that 95% of employees working in an environment where the manager is sensitive to work and personal life—including informal flexibility— feel motivated to exceed expectations, compared to 80% of employees in environments where the manager is not sensitive to needs for informal flexibility. (24)
"At Bristol-Myers Squibb, 14% of employees have a formal flexible work arrangement. Of the remaining, 67% say they have informal flexibility. When asked about the importance of informal flexibility in terms of their intention to continue working at the company, the response is resounding: 71% say that it is 'very important.' Again, women place even greater importance on informal flexibility; 78% of women say it is 'very important' to their staying, compared to 65% of men. The retention effect is especially strong for women in management: 84% say informal flexibility helps keep them at the company." (24)
DISCIPLINARY CONTENT
Posted on: Queens group


Forests
he eastern United States and Canada are well-known for gorgeous autumn colors.
NAVIGATING CONTEXT
Posted on: #iteachmsu


Biochemistry -test
Biochemistry, study of the chemical substances and processes that occur in plants, animals, and microorganisms and of the changes they undergo during development and life. It deals with the chemistry of life, and as such it draws on the techniques of analytical, organic, and physical chemistry, as well as those of physiologists concerned with the molecular basis of vital processes. All chemical changes within the organism—either the degradation of substances, generally to gain necessary energy, or the buildup of complex molecules necessary for life processes—are collectively termed metabolism. These chemical changes depend on the action of organic catalysts known as enzymes, and enzymes, in turn, depend for their existence on the genetic apparatus of the cell. It is not surprising, therefore, that biochemistry enters into the investigation of chemical changes in disease, drug action, and other aspects of medicine, as well as in nutrition, genetics, and agriculture.
The term biochemistry is synonymous with two somewhat older terms: physiological chemistry and biological chemistry. Those aspects of biochemistry that deal with the chemistry and function of very large molecules (e.g., proteins and nucleic acids) are often grouped under the term molecular biology. Biochemistry is a young science, having been known under that term only since about 1900. Its origins, however, can be traced much further back; its early history is part of the early history of both physiology and chemistry.
Historical background
The particularly significant past events in biochemistry have been concerned with placing biological phenomena on firm chemical foundations.
Before chemistry could contribute adequately to medicine and agriculture, however, it had to free itself from immediate practical demands in order to become a pure science. This happened in the period from about 1650 to 1780, starting with the work of Robert Boyle and culminating in that of Antoine-Laurent Lavoisier, the father of modern chemistry. Boyle questioned the basis of the chemical theory of his day and taught that the proper object of chemistry was to determine the composition of substances. His contemporary John Mayow observed the fundamental analogy between the respiration of an animal and the burning, or oxidation, of organic matter in air. Then, when Lavoisier carried out his fundamental studies on chemical oxidation, grasping the true nature of the process, he also showed, quantitatively, the similarity between chemical oxidation and the respiratory process. Photosynthesis was another biological phenomenon that occupied the attention of the chemists of the late 18th century. The demonstration, through the combined work of Joseph Priestley, Jan Ingenhousz, and Jean Senebier, that photosynthesis is essentially the reverse of respiration was a milestone in the development of biochemical thought.
Get unlimited ad-free access to all Britannica’s trusted content.
Start Your Free Trial Today
In spite of these early fundamental discoveries, rapid progress in biochemistry had to wait upon the development of structural organic chemistry, one of the great achievements of 19th-century science. A living organism contains many thousands of different chemical compounds. The elucidation of the chemical transformations undergone by these compounds within the living cell is a central problem of biochemistry. Clearly, the determination of the molecular structure of the organic substances present in living cells had to precede the study of the cellular mechanisms, whereby these substances are synthesized and degraded.
There are few sharp boundaries in science, and the boundaries between organic and physical chemistry, on the one hand, and biochemistry, on the other, have always shown much overlap. Biochemistry has borrowed the methods and theories of organic and physical chemistry and applied them to physiological problems. Progress in this path was at first impeded by a stubborn misconception in scientific thinking—the error of supposing that the transformations undergone by matter in the living organism were not subject to the chemical and physical laws that applied to inanimate substances and that consequently these “vital” phenomena could not be described in ordinary chemical or physical terms. Such an attitude was taken by the vitalists, who maintained that natural products formed by living organisms could never be synthesized by ordinary chemical means. The first laboratory synthesis of an organic compound, urea, by Friedrich Wöhler in 1828, was a blow to the vitalists but not a decisive one. They retreated to new lines of defense, arguing that urea was only an excretory substance—a product of breakdown and not of synthesis. The success of the organic chemists in synthesizing many natural products forced further retreats of the vitalists. It is axiomatic in modern biochemistry that the chemical laws that apply to inanimate materials are equally valid within the living cell.
Advertisement
At the same time that progress was being impeded by a misplaced kind of reverence for living phenomena, the practical needs of man operated to spur the progress of the new science. As organic and physical chemistry erected an imposing body of theory in the 19th century, the needs of the physician, the pharmacist, and the agriculturalist provided an ever-present stimulus for the application of the new discoveries of chemistry to various urgent practical problems.
Two outstanding figures of the 19th century, Justus von Liebig and Louis Pasteur, were particularly responsible for dramatizing the successful application of chemistry to the study of biology. Liebig studied chemistry in Paris and carried back to Germany the inspiration gained by contact with the former students and colleagues of Lavoisier. He established at Giessen a great teaching and research laboratory, one of the first of its kind, which drew students from all over Europe.
Besides putting the study of organic chemistry on a firm basis, Liebig engaged in extensive literary activity, attracting the attention of all scientists to organic chemistry and popularizing it for the layman as well. His classic works, published in the 1840s, had a profound influence on contemporary thought. Liebig described the great chemical cycles in nature. He pointed out that animals would disappear from the face of the Earth if it were not for the photosynthesizing plants, since animals require for their nutrition the complex organic compounds that can be synthesized only by plants. The animal excretions and the animal body after death are also converted by a process of decay to simple products that can be re-utilized only by plants.
In contrast with animals, green plants require for their growth only carbon dioxide, water, mineral salts, and sunlight. The minerals must be obtained from the soil, and the fertility of the soil depends on its ability to furnish the plants with these essential nutrients. But the soil is depleted of these materials by the removal of successive crops; hence the need for fertilizers. Liebig pointed out that chemical analysis of plants could serve as a guide to the substances that should be present in fertilizers. Agricultural chemistry as an applied science was thus born.
In his analysis of fermentation, putrefaction, and infectious disease, Liebig was less fortunate. He admitted the similarity of these phenomena but refused to admit that living organisms might function as the causative agents. It remained for Pasteur to clarify that matter. In the 1860s Pasteur proved that various yeasts and bacteria were responsible for “ferments,” substances that caused fermentation and, in some cases, disease. He also demonstrated the usefulness of chemical methods in studying these tiny organisms and was the founder of what came to be called bacteriology.
Later, in 1877, Pasteur’s ferments were designated as enzymes, and, in 1897, the German chemist E. Buchner clearly showed that fermentation could occur in a press juice of yeast, devoid of living cells. Thus a life process of cells was reduced by analysis to a nonliving system of enzymes. The chemical nature of enzymes remained obscure until 1926, when the first pure crystalline enzyme (urease) was isolated. This enzyme and many others subsequently isolated proved to be proteins, which had already been recognized as high-molecular-weight chains of subunits called amino acids.
The mystery of how minute amounts of dietary substances known as the vitamins prevent diseases such as beriberi, scurvy, and pellagra became clear in 1935, when riboflavin (vitamin B2) was found to be an integral part of an enzyme. Subsequent work has substantiated the concept that many vitamins are essential in the chemical reactions of the cell by virtue of their role in enzymes.
In 1929 the substance adenosine triphosphate (ATP) was isolated from muscle. Subsequent work demonstrated that the production of ATP was associated with respiratory (oxidative) processes in the cell. In 1940 F.A. Lipmann proposed that ATP is the common form of energy exchange in many cells, a concept now thoroughly documented. ATP has been shown also to be a primary energy source for muscular contraction.
The use of radioactive isotopes of chemical elements to trace the pathway of substances in the animal body was initiated in 1935 by two U.S. chemists, R. Schoenheimer and D. Rittenberg. That technique provided one of the single most important tools for investigating the complex chemical changes that occur in life processes. At about the same time, other workers localized the sites of metabolic reactions by ingenious technical advances in the studies of organs, tissue slices, cell mixtures, individual cells, and, finally, individual cell constituents, such as nuclei, mitochondria, ribosomes, lysosomes, and membranes.
In 1869 a substance was isolated from the nuclei of pus cells and was called nucleic acid, which later proved to be deoxyribonucleic acid (DNA), but it was not until 1944 that the significance of DNA as genetic material was revealed, when bacterial DNA was shown to change the genetic matter of other bacterial cells. Within a decade of that discovery, the double helix structure of DNA was proposed by Watson and Crick, providing a firm basis for understanding how DNA is involved in cell division and in maintaining genetic characteristics.
Advances have continued since that time, with such landmark events as the first chemical synthesis of a protein, the detailed mapping of the arrangement of atoms in some enzymes, and the elucidation of intricate mechanisms of metabolic regulation, including the molecular action of hormones.
SpaceNext50
Biochemistry
Quick Facts
key people
George P. Smith
Gregory P. Winter
Joachim Frank
Justus, baron von Liebig
Frederick Sanger
Bruce Ames
Melvin Calvin
Aleksandr Oparin
J. Craig Venter
Dorothy Maud Wrinch
related topics
Chemistry
Biology
Immunochemistry
Key-lock hypothesis
Histochemistry
The term biochemistry is synonymous with two somewhat older terms: physiological chemistry and biological chemistry. Those aspects of biochemistry that deal with the chemistry and function of very large molecules (e.g., proteins and nucleic acids) are often grouped under the term molecular biology. Biochemistry is a young science, having been known under that term only since about 1900. Its origins, however, can be traced much further back; its early history is part of the early history of both physiology and chemistry.
Historical background
The particularly significant past events in biochemistry have been concerned with placing biological phenomena on firm chemical foundations.
Before chemistry could contribute adequately to medicine and agriculture, however, it had to free itself from immediate practical demands in order to become a pure science. This happened in the period from about 1650 to 1780, starting with the work of Robert Boyle and culminating in that of Antoine-Laurent Lavoisier, the father of modern chemistry. Boyle questioned the basis of the chemical theory of his day and taught that the proper object of chemistry was to determine the composition of substances. His contemporary John Mayow observed the fundamental analogy between the respiration of an animal and the burning, or oxidation, of organic matter in air. Then, when Lavoisier carried out his fundamental studies on chemical oxidation, grasping the true nature of the process, he also showed, quantitatively, the similarity between chemical oxidation and the respiratory process. Photosynthesis was another biological phenomenon that occupied the attention of the chemists of the late 18th century. The demonstration, through the combined work of Joseph Priestley, Jan Ingenhousz, and Jean Senebier, that photosynthesis is essentially the reverse of respiration was a milestone in the development of biochemical thought.
Get unlimited ad-free access to all Britannica’s trusted content.
Start Your Free Trial Today
In spite of these early fundamental discoveries, rapid progress in biochemistry had to wait upon the development of structural organic chemistry, one of the great achievements of 19th-century science. A living organism contains many thousands of different chemical compounds. The elucidation of the chemical transformations undergone by these compounds within the living cell is a central problem of biochemistry. Clearly, the determination of the molecular structure of the organic substances present in living cells had to precede the study of the cellular mechanisms, whereby these substances are synthesized and degraded.
There are few sharp boundaries in science, and the boundaries between organic and physical chemistry, on the one hand, and biochemistry, on the other, have always shown much overlap. Biochemistry has borrowed the methods and theories of organic and physical chemistry and applied them to physiological problems. Progress in this path was at first impeded by a stubborn misconception in scientific thinking—the error of supposing that the transformations undergone by matter in the living organism were not subject to the chemical and physical laws that applied to inanimate substances and that consequently these “vital” phenomena could not be described in ordinary chemical or physical terms. Such an attitude was taken by the vitalists, who maintained that natural products formed by living organisms could never be synthesized by ordinary chemical means. The first laboratory synthesis of an organic compound, urea, by Friedrich Wöhler in 1828, was a blow to the vitalists but not a decisive one. They retreated to new lines of defense, arguing that urea was only an excretory substance—a product of breakdown and not of synthesis. The success of the organic chemists in synthesizing many natural products forced further retreats of the vitalists. It is axiomatic in modern biochemistry that the chemical laws that apply to inanimate materials are equally valid within the living cell.
Advertisement
At the same time that progress was being impeded by a misplaced kind of reverence for living phenomena, the practical needs of man operated to spur the progress of the new science. As organic and physical chemistry erected an imposing body of theory in the 19th century, the needs of the physician, the pharmacist, and the agriculturalist provided an ever-present stimulus for the application of the new discoveries of chemistry to various urgent practical problems.
Two outstanding figures of the 19th century, Justus von Liebig and Louis Pasteur, were particularly responsible for dramatizing the successful application of chemistry to the study of biology. Liebig studied chemistry in Paris and carried back to Germany the inspiration gained by contact with the former students and colleagues of Lavoisier. He established at Giessen a great teaching and research laboratory, one of the first of its kind, which drew students from all over Europe.
Besides putting the study of organic chemistry on a firm basis, Liebig engaged in extensive literary activity, attracting the attention of all scientists to organic chemistry and popularizing it for the layman as well. His classic works, published in the 1840s, had a profound influence on contemporary thought. Liebig described the great chemical cycles in nature. He pointed out that animals would disappear from the face of the Earth if it were not for the photosynthesizing plants, since animals require for their nutrition the complex organic compounds that can be synthesized only by plants. The animal excretions and the animal body after death are also converted by a process of decay to simple products that can be re-utilized only by plants.
In contrast with animals, green plants require for their growth only carbon dioxide, water, mineral salts, and sunlight. The minerals must be obtained from the soil, and the fertility of the soil depends on its ability to furnish the plants with these essential nutrients. But the soil is depleted of these materials by the removal of successive crops; hence the need for fertilizers. Liebig pointed out that chemical analysis of plants could serve as a guide to the substances that should be present in fertilizers. Agricultural chemistry as an applied science was thus born.
In his analysis of fermentation, putrefaction, and infectious disease, Liebig was less fortunate. He admitted the similarity of these phenomena but refused to admit that living organisms might function as the causative agents. It remained for Pasteur to clarify that matter. In the 1860s Pasteur proved that various yeasts and bacteria were responsible for “ferments,” substances that caused fermentation and, in some cases, disease. He also demonstrated the usefulness of chemical methods in studying these tiny organisms and was the founder of what came to be called bacteriology.
Later, in 1877, Pasteur’s ferments were designated as enzymes, and, in 1897, the German chemist E. Buchner clearly showed that fermentation could occur in a press juice of yeast, devoid of living cells. Thus a life process of cells was reduced by analysis to a nonliving system of enzymes. The chemical nature of enzymes remained obscure until 1926, when the first pure crystalline enzyme (urease) was isolated. This enzyme and many others subsequently isolated proved to be proteins, which had already been recognized as high-molecular-weight chains of subunits called amino acids.
The mystery of how minute amounts of dietary substances known as the vitamins prevent diseases such as beriberi, scurvy, and pellagra became clear in 1935, when riboflavin (vitamin B2) was found to be an integral part of an enzyme. Subsequent work has substantiated the concept that many vitamins are essential in the chemical reactions of the cell by virtue of their role in enzymes.
In 1929 the substance adenosine triphosphate (ATP) was isolated from muscle. Subsequent work demonstrated that the production of ATP was associated with respiratory (oxidative) processes in the cell. In 1940 F.A. Lipmann proposed that ATP is the common form of energy exchange in many cells, a concept now thoroughly documented. ATP has been shown also to be a primary energy source for muscular contraction.
The use of radioactive isotopes of chemical elements to trace the pathway of substances in the animal body was initiated in 1935 by two U.S. chemists, R. Schoenheimer and D. Rittenberg. That technique provided one of the single most important tools for investigating the complex chemical changes that occur in life processes. At about the same time, other workers localized the sites of metabolic reactions by ingenious technical advances in the studies of organs, tissue slices, cell mixtures, individual cells, and, finally, individual cell constituents, such as nuclei, mitochondria, ribosomes, lysosomes, and membranes.
In 1869 a substance was isolated from the nuclei of pus cells and was called nucleic acid, which later proved to be deoxyribonucleic acid (DNA), but it was not until 1944 that the significance of DNA as genetic material was revealed, when bacterial DNA was shown to change the genetic matter of other bacterial cells. Within a decade of that discovery, the double helix structure of DNA was proposed by Watson and Crick, providing a firm basis for understanding how DNA is involved in cell division and in maintaining genetic characteristics.
Advances have continued since that time, with such landmark events as the first chemical synthesis of a protein, the detailed mapping of the arrangement of atoms in some enzymes, and the elucidation of intricate mechanisms of metabolic regulation, including the molecular action of hormones.
SpaceNext50
Biochemistry
Quick Facts
key people
George P. Smith
Gregory P. Winter
Joachim Frank
Justus, baron von Liebig
Frederick Sanger
Bruce Ames
Melvin Calvin
Aleksandr Oparin
J. Craig Venter
Dorothy Maud Wrinch
related topics
Chemistry
Biology
Immunochemistry
Key-lock hypothesis
Histochemistry
Posted on: #iteachmsu

Education - 2
What is the CPD framework for teacher educators?
The framework describes the overall competence and the kinds of professional knowledge, understanding and skills associated with the role of a teacher educator. It is used to help teacher educators, and those involved with the professional development of teacher educators, to think about and further develop the overall competence, knowledge, understanding and skills required for effective and supportive teacher education. It is based on an extensive survey of research into teacher educator competence in a wide range of educational settings and covering a range of teacher educator roles. It has been refined through feedback provided by senior academics and teacher educators from around the world and working in different areas of teacher education.
The framework describes the overall competence and the kinds of professional knowledge, understanding and skills associated with the role of a teacher educator. It is used to help teacher educators, and those involved with the professional development of teacher educators, to think about and further develop the overall competence, knowledge, understanding and skills required for effective and supportive teacher education. It is based on an extensive survey of research into teacher educator competence in a wide range of educational settings and covering a range of teacher educator roles. It has been refined through feedback provided by senior academics and teacher educators from around the world and working in different areas of teacher education.
Posted on: #iteachmsu


research
This article is about the search for knowledge. For other uses, see Research (disambiguation).
"Researcher" redirects here. For other uses, see Researcher (disambiguation).
"Original research" redirects here. For the Wikipedia prohibition against user-generated, unpublished research
"Researcher" redirects here. For other uses, see Researcher (disambiguation).
"Original research" redirects here. For the Wikipedia prohibition against user-generated, unpublished research
PEDAGOGICAL DESIGN
Posted on: #iteachmsu


Natural resources gifted by god --- Edited
Natural resources are the raw materials and sources of energy that we use. Petrol, metals, soil, sand, wind, water, and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Natural resources are the raw materials and sources of energy that we use.
Petrol, metals, soil, sand, wind, water and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Think about the relationship between natural resources and manufactured products. In essence, we call them “natural” resources because they are things human society uses that are created (or were created in the case of fossil fuels) without human intervention.
Perpetually Renewable Resources
Perpetually renewable resources are the easiest resources to understand; these are natural resources that are constantly replenished by the Sun’s and Earth’s natural processes. For example, every day the sun delivers an average of 198 Watts of energy to every square meter (m
) of the Earth’s surface. For comparison a standard incandescent light bulb in a bedside lamp uses 40 Watts, or a 100kg person climbing a step in 2 seconds uses roughly 200 Watts. Every day without fail for the last 5 billion years (plus or minus a few hundred million years) the Sun has delivered this solar energy.
Together with geothermal energy (heat from the Earth’s interior), the Sun’s perpetual energy powers the winds, ocean currents, precipitation and most of the Earth’s plant life. Solar and geothermal natural resources currently energise a significant and growing percentage of many nations’ electrical grids. It is perpetually renewable in the sense that no matter how much we use in terms of human time-scales (e.g decades to millennia), the Sun and the Earth will always make more.
Intermediate Renewable Resources
Intermediate renewable resources are only renewable resources if we don’t use them too quickly. They are resources such as freshwater, soil, crops and trees for timber. If we didn’t use them, they would be perpetually renewable, but because they require time (on human time-scales) to regenerate or grow, we can overuse them until they are no longer available.
Freshwater is a great example of an intermediate renewable resource. Through the water cycle, the sun evaporates water from the surface of saltwater oceans that travels over land and falls back to earth as freshwater rain. This rain fills the lakes, rivers and aquifers we use for agriculture, industry and drinking water. If we use this freshwater at the same rate as the rain recharging it, then we won’t run out. If we use the freshwater faster than it recharges, then we will. Intermediate renewable resources must be carefully managed to ensure they are not depleted.
Non-renewable Resources
The last category of natural resources are the non-renewables. These are resources that will not regenerate on human time-scales. Once they have been depleted they will no longer be available and no more will be made. The most common examples of non-renewable resources are fossil fuels, so-called because most were created by processes that take millions of years. Fossil fuels include crude oil, natural gas, coal and uranium. Other non-renewable resources include metals, lithium and rare-Earth elements (REE’s), but it’s important to remember that while we may eventually run out of mineable metals and REE’s, with careful waste management, these can be recovered through recycling. However, it is not the same for fossil fuels as using them for energy alters their chemistry so they are no longer useful.
Natural resources are the raw materials and sources of energy that we use.
Petrol, metals, soil, sand, wind, water and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Think about the relationship between natural resources and manufactured products. In essence, we call them “natural” resources because they are things human society uses that are created (or were created in the case of fossil fuels) without human intervention.
Perpetually Renewable Resources
Perpetually renewable resources are the easiest resources to understand; these are natural resources that are constantly replenished by the Sun’s and Earth’s natural processes. For example, every day the sun delivers an average of 198 Watts of energy to every square meter (m
) of the Earth’s surface. For comparison a standard incandescent light bulb in a bedside lamp uses 40 Watts, or a 100kg person climbing a step in 2 seconds uses roughly 200 Watts. Every day without fail for the last 5 billion years (plus or minus a few hundred million years) the Sun has delivered this solar energy.
Together with geothermal energy (heat from the Earth’s interior), the Sun’s perpetual energy powers the winds, ocean currents, precipitation and most of the Earth’s plant life. Solar and geothermal natural resources currently energise a significant and growing percentage of many nations’ electrical grids. It is perpetually renewable in the sense that no matter how much we use in terms of human time-scales (e.g decades to millennia), the Sun and the Earth will always make more.
Intermediate Renewable Resources
Intermediate renewable resources are only renewable resources if we don’t use them too quickly. They are resources such as freshwater, soil, crops and trees for timber. If we didn’t use them, they would be perpetually renewable, but because they require time (on human time-scales) to regenerate or grow, we can overuse them until they are no longer available.
Freshwater is a great example of an intermediate renewable resource. Through the water cycle, the sun evaporates water from the surface of saltwater oceans that travels over land and falls back to earth as freshwater rain. This rain fills the lakes, rivers and aquifers we use for agriculture, industry and drinking water. If we use this freshwater at the same rate as the rain recharging it, then we won’t run out. If we use the freshwater faster than it recharges, then we will. Intermediate renewable resources must be carefully managed to ensure they are not depleted.
Non-renewable Resources
The last category of natural resources are the non-renewables. These are resources that will not regenerate on human time-scales. Once they have been depleted they will no longer be available and no more will be made. The most common examples of non-renewable resources are fossil fuels, so-called because most were created by processes that take millions of years. Fossil fuels include crude oil, natural gas, coal and uranium. Other non-renewable resources include metals, lithium and rare-Earth elements (REE’s), but it’s important to remember that while we may eventually run out of mineable metals and REE’s, with careful waste management, these can be recovered through recycling. However, it is not the same for fossil fuels as using them for energy alters their chemistry so they are no longer useful.
Authored by: Saarth
Disciplinary Content
Posted on: #iteachmsu


image in description
image in description
Types of people who will benefit from the Eisenhower Matrix:
People in leadership positions
Critical thinkers
4. Parkinson’s Law
British historian Cyril Northcote Parkinson became famous for the phrase “work expands so as to fill the time available for its completion.” In other words, the amount of time you give yourself to complete a specific task is the amount of time it will take you to complete that task.
How it works:
This is not a time management technique per se. It’s a law that, when understood, can be applied as one of the most beneficial time management methods out there—but you will have to put in the work. That means working more efficiently in shorter bursts of time. Here are some time management tips:
Try working without a computer charger. This will force you to finish a project before your computer dies.
Get it done early. Instead of finishing an essay by midnight, try to get it done by noon.
Set a deadline. Give yourself a set time to do something—and then cut it in half.
Limit time for tasks. Give yourself only 20 minutes in the morning to answer emails.
Types of people this works for:
Procrastinators
People who work well under pressure
5. Time Blocking Method
Inventor Elon Musk is known for being productive. He manages his time so efficiently that he can work over 80 hours a week and still make time for himself. What’s his secret? Time blocking.
Types of people who will benefit from the Eisenhower Matrix:
People in leadership positions
Critical thinkers
4. Parkinson’s Law
British historian Cyril Northcote Parkinson became famous for the phrase “work expands so as to fill the time available for its completion.” In other words, the amount of time you give yourself to complete a specific task is the amount of time it will take you to complete that task.
How it works:
This is not a time management technique per se. It’s a law that, when understood, can be applied as one of the most beneficial time management methods out there—but you will have to put in the work. That means working more efficiently in shorter bursts of time. Here are some time management tips:
Try working without a computer charger. This will force you to finish a project before your computer dies.
Get it done early. Instead of finishing an essay by midnight, try to get it done by noon.
Set a deadline. Give yourself a set time to do something—and then cut it in half.
Limit time for tasks. Give yourself only 20 minutes in the morning to answer emails.
Types of people this works for:
Procrastinators
People who work well under pressure
5. Time Blocking Method
Inventor Elon Musk is known for being productive. He manages his time so efficiently that he can work over 80 hours a week and still make time for himself. What’s his secret? Time blocking.
Posted by: Venturit Super Admin
Navigating Context
Posted on: #iteachmsu


Getting Started
What is the #iteachmsu Commons?
Welcome to the #iteachmsu Commons
You teach MSU. We, the Academic Advancement Network, The Graduate School, and The Hub for Innovation in Learning and Technology, believe that a wide educator community (faculty, TAs, ULAs, instructional designers, academic advisors, et al.) makes learning happen across MSU. But, on such a large campus, it can be difficult to fully recognize and leverage this community’s teaching and learning innovations. To address this challenge, the #iteachmsu Commons provides an educator-driven space for sharing teaching resources, connecting across educator networks, and growing teaching practice.
#iteachmsu Commons content may be discipline-specific or transdisciplinary, but will always be anchored in teaching competency areas. You will find short posts, blog-like articles, curated playlists, and a campus-wide teaching and learning events calendar. We cultivate this commons across spaces. And through your engagement, we will continue to nurture a culture of teaching and learning across MSU and beyond.
How to login
To begin creating content of your own on the #iteachmsu Commons, simply click the green Login button in the upper right hand corner of the screen. Your account will automatically be provisioned after successfully logging into the MSU Net ID login prompt. Currently, only authenticated MSU faculty, staff and students can create content on the #iteachmsu Commons. However, external users are free to browse and share public facing content without logging into the site.
Where to start
If you are looking for brief instructive videos on the core functionality of the site, take a look at our Getting Started playlist. After viewing each one of the video tutorials on the playlist, you will receive a Contributor badge which will display on your profile
What Are the #iteachmsu Commons Policies?
Part of the mission of the #iteachmsu Commons is to provide space for sharing, reflecting, and learning for all educators on our campus wherever they are in their teaching development. The commons is designed to encourage these types of interactions and reflect policies outlined by the MSU Faculty Senate. We maintain the right to remove any post that violates guidelines as outlined here and by MSU. To maintain a useful and safer commons, we ask that you:
Follow the MSU Guidelines for Social Media.
Engage across the #iteachmsu commons in a civil and respectful manner. Content may be moderated in accordance with the MSU Guidelines for Social Media.Do not share private or confidential information via shared content on the #iteachmsu Commons.
Content posted on the #iteachmsu Commons is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International license. Learn more about this licensing here. Posted comments, images, etc. on the #iteachmsu Commons do not necessarily represent the views of Michigan State University or the #iteachmsu Commons Team. Links to external, non-#iteachmsu Commons content do not constitute official endorsement by, or necessarily represent the views of, the #iteachmsu Commons or Michigan State University.
Other important policies:
MSU's Web Accessibility Statement
MSU's Privacy Statement
What if I Have #iteachmsu Commons Questions and/or Feedback?
If you have any concerns about #iteachmsu Commons content, please email us at iteach@msu.edu. We welcome all feedback and thank you for your help in promoting a safer, vibrant and respectful community.
Stay up to date with the #iteachmsu Digest
Welcome to the #iteachmsu Commons
You teach MSU. We, the Academic Advancement Network, The Graduate School, and The Hub for Innovation in Learning and Technology, believe that a wide educator community (faculty, TAs, ULAs, instructional designers, academic advisors, et al.) makes learning happen across MSU. But, on such a large campus, it can be difficult to fully recognize and leverage this community’s teaching and learning innovations. To address this challenge, the #iteachmsu Commons provides an educator-driven space for sharing teaching resources, connecting across educator networks, and growing teaching practice.
#iteachmsu Commons content may be discipline-specific or transdisciplinary, but will always be anchored in teaching competency areas. You will find short posts, blog-like articles, curated playlists, and a campus-wide teaching and learning events calendar. We cultivate this commons across spaces. And through your engagement, we will continue to nurture a culture of teaching and learning across MSU and beyond.
How to login
To begin creating content of your own on the #iteachmsu Commons, simply click the green Login button in the upper right hand corner of the screen. Your account will automatically be provisioned after successfully logging into the MSU Net ID login prompt. Currently, only authenticated MSU faculty, staff and students can create content on the #iteachmsu Commons. However, external users are free to browse and share public facing content without logging into the site.
Where to start
If you are looking for brief instructive videos on the core functionality of the site, take a look at our Getting Started playlist. After viewing each one of the video tutorials on the playlist, you will receive a Contributor badge which will display on your profile
What Are the #iteachmsu Commons Policies?
Part of the mission of the #iteachmsu Commons is to provide space for sharing, reflecting, and learning for all educators on our campus wherever they are in their teaching development. The commons is designed to encourage these types of interactions and reflect policies outlined by the MSU Faculty Senate. We maintain the right to remove any post that violates guidelines as outlined here and by MSU. To maintain a useful and safer commons, we ask that you:
Follow the MSU Guidelines for Social Media.
Engage across the #iteachmsu commons in a civil and respectful manner. Content may be moderated in accordance with the MSU Guidelines for Social Media.Do not share private or confidential information via shared content on the #iteachmsu Commons.
Content posted on the #iteachmsu Commons is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International license. Learn more about this licensing here. Posted comments, images, etc. on the #iteachmsu Commons do not necessarily represent the views of Michigan State University or the #iteachmsu Commons Team. Links to external, non-#iteachmsu Commons content do not constitute official endorsement by, or necessarily represent the views of, the #iteachmsu Commons or Michigan State University.
Other important policies:
MSU's Web Accessibility Statement
MSU's Privacy Statement
What if I Have #iteachmsu Commons Questions and/or Feedback?
If you have any concerns about #iteachmsu Commons content, please email us at iteach@msu.edu. We welcome all feedback and thank you for your help in promoting a safer, vibrant and respectful community.
Stay up to date with the #iteachmsu Digest
Authored by: Admin #iteachmsu
Navigating Context
Posted on: #iteachmsu


Chemical testing overview
Overview
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.Description is the fiction-writing mode for transmitting a mental image of the particulars of a story. Together with dialogue, narration, exposition, and summarization, description is one of the most widely recognized of the fiction-writing modes. As stated in Writing from A to Z, edited by Kirk Polking, description is more than the amassing of details; it is bringing something to life by carefully choosing and arranging words and phrases to produce the desired effect.[6] The most appropriate and effective techniques for presenting description are a matter of ongoing discussion among writers and writing coaches.
Authored by: Chathuri
Assessing Learning
Posted on: #iteachmsu
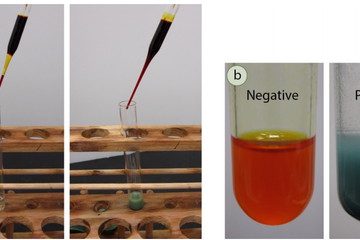

Chemical testing overview
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color
Authored by: Chathuri
Assessing Learning
Posted on: #iteachmsu


By Chathu: Fish Farming article
Fish farming is an ideal business idea for investors with available land, and it doesn’t always require a large body of water. You can start a fish farm either by creating fish ponds or investing in fish tanks; it’s a highly scalable business idea. Once you have the proper knowledge of fish raising, you will be able to decide the type of fish to raise. According to FinModelsLab, a well-run fish farm can produce a return on investment (ROI) of 15% to 40% annually, with many fish farmers achieving a full return on their investment within three to five years.
Fish such as tilapia, cod, trout, and catfish are popular because they are easy to raise and in high demand. Small-scale farms are the usual suppliers of fish in their local supermarkets and restaurants.
Other popular varieties of commercially-raised fish include:
Yellow Perch
Eel
Grass Carp
Tuna
Salmon
Not all fish are raised for food. Goldfish and koi are popular fish to farm as well. For non-food fish, focus on cultivating premium species that will fetch a higher price when you sell. Whether you are raising fish for food or not, adopting sustainable farming practices will be better for both your business branding and your long-term operational costs.
The decision as to which fish you want to raise will ultimately rely on your skill, financial capacity, market demand, and agro-climatic condition. This refers to the normal soil types, rainfall, temperature, and water availability that affect the type of vegetation in the area.
Fish such as tilapia, cod, trout, and catfish are popular because they are easy to raise and in high demand. Small-scale farms are the usual suppliers of fish in their local supermarkets and restaurants.
Other popular varieties of commercially-raised fish include:
Yellow Perch
Eel
Grass Carp
Tuna
Salmon
Not all fish are raised for food. Goldfish and koi are popular fish to farm as well. For non-food fish, focus on cultivating premium species that will fetch a higher price when you sell. Whether you are raising fish for food or not, adopting sustainable farming practices will be better for both your business branding and your long-term operational costs.
The decision as to which fish you want to raise will ultimately rely on your skill, financial capacity, market demand, and agro-climatic condition. This refers to the normal soil types, rainfall, temperature, and water availability that affect the type of vegetation in the area.
Posted by: Chathuri Hewapathirana 1
Posted on: #iteachmsu

Article450
5 days ago - A girl answering (= matching) the description of the missing teenager was spotted in Glasgow. ... These examples are from the Cambridge English Corpus and from sources on the web. Any opinions in the examples do not represent the opinion of the Cambridge Dictionary editors or of ..
5 days ago - A girl answering (= matching) the description of the missing teenager was spotted in Glasgow. ... These examples are from the Cambridge English Corpus and from sources on the web. Any opinions in the examples do not represent the opinion of the Cambridge Dictionary editors or of ....
5 days ago - A girl answering (= matching) the description of the missing teenager was spotted in Glasgow. ... These examples are from the Cambridge English Corpus and from sources on the web. Any opinions in the examples do not represent the opinion of the Cambridge Dictionary editors or of ....
Authored by: kemil
Assessing Learning
Posted on: #iteachmsu


Chemical testing overview
Overview
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color
Authored by: Chathuri
Assessing Learning
Posted on: #iteachmsu


Natural resources are the raw materials and sources of energy that we use. Petrol, metals, soil, sand, wind, water, and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Natural resources are the raw materials and sources of energy that we use.
Petrol, metals, soil, sand, wind, water and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Think about the relationship between natural resources and manufactured products. In essence, we call them “natural” resources because they are things human society uses that are created (or were created in the case of fossil fuels) without human intervention.
Perpetually Renewable Resources
Perpetually renewable resources are the easiest resources to understand; these are natural resources that are constantly replenished by the Sun’s and Earth’s natural processes. For example, every day the sun delivers an average of 198 Watts of energy to every square meter (m
) of the Earth’s surface. For comparison a standard incandescent light bulb in a bedside lamp uses 40 Watts, or a 100kg person climbing a step in 2 seconds uses roughly 200 Watts. Every day without fail for the last 5 billion years (plus or minus a few hundred million years) the Sun has delivered this solar energy.
Together with geothermal energy (heat from the Earth’s interior), the Sun’s perpetual energy powers the winds, ocean currents, precipitation and most of the Earth’s plant life. Solar and geothermal natural resources currently energise a significant and growing percentage of many nations’ electrical grids. It is perpetually renewable in the sense that no matter how much we use in terms of human time-scales (e.g decades to millennia), the Sun and the Earth will always make more.
Intermediate Renewable Resources
Intermediate renewable resources are only renewable resources if we don’t use them too quickly. They are resources such as freshwater, soil, crops and trees for timber. If we didn’t use them, they would be perpetually renewable, but because they require time (on human time-scales) to regenerate or grow, we can overuse them until they are no longer available.
Freshwater is a great example of an intermediate renewable resource. Through the water cycle, the sun evaporates water from the surface of saltwater oceans that travels over land and falls back to earth as freshwater rain. This rain fills the lakes, rivers and aquifers we use for agriculture, industry and drinking water. If we use this freshwater at the same rate as the rain recharging it, then we won’t run out. If we use the freshwater faster than it recharges, then we will. Intermediate renewable resources must be carefully managed to ensure they are not depleted.
Non-renewable Resources
The last category of natural resources are the non-renewables. These are resources that will not regenerate on human time-scales. Once they have been depleted they will no longer be available and no more will be made. The most common examples of non-renewable resources are fossil fuels, so-called because most were created by processes that take millions of years. Fossil fuels include crude oil, natural gas, coal and uranium. Other non-renewable resources include metals, lithium and rare-Earth elements (REE’s), but it’s important to remember that while we may eventually run out of mineable metals and REE’s, with careful waste management, these can be recovered through recycling. However, it is not the same for fossil fuels as using them for energy alters their chemistry so they are no longer useful.
Natural resources are the raw materials and sources of energy that we use.
Petrol, metals, soil, sand, wind, water and everything in between are natural resources. Manufactured items such as plastic, sheet metal, fabrics, microchips, electricity and concrete are not natural resources, but are most definitely derived from natural resources.
Think about the relationship between natural resources and manufactured products. In essence, we call them “natural” resources because they are things human society uses that are created (or were created in the case of fossil fuels) without human intervention.
Perpetually Renewable Resources
Perpetually renewable resources are the easiest resources to understand; these are natural resources that are constantly replenished by the Sun’s and Earth’s natural processes. For example, every day the sun delivers an average of 198 Watts of energy to every square meter (m
) of the Earth’s surface. For comparison a standard incandescent light bulb in a bedside lamp uses 40 Watts, or a 100kg person climbing a step in 2 seconds uses roughly 200 Watts. Every day without fail for the last 5 billion years (plus or minus a few hundred million years) the Sun has delivered this solar energy.
Together with geothermal energy (heat from the Earth’s interior), the Sun’s perpetual energy powers the winds, ocean currents, precipitation and most of the Earth’s plant life. Solar and geothermal natural resources currently energise a significant and growing percentage of many nations’ electrical grids. It is perpetually renewable in the sense that no matter how much we use in terms of human time-scales (e.g decades to millennia), the Sun and the Earth will always make more.
Intermediate Renewable Resources
Intermediate renewable resources are only renewable resources if we don’t use them too quickly. They are resources such as freshwater, soil, crops and trees for timber. If we didn’t use them, they would be perpetually renewable, but because they require time (on human time-scales) to regenerate or grow, we can overuse them until they are no longer available.
Freshwater is a great example of an intermediate renewable resource. Through the water cycle, the sun evaporates water from the surface of saltwater oceans that travels over land and falls back to earth as freshwater rain. This rain fills the lakes, rivers and aquifers we use for agriculture, industry and drinking water. If we use this freshwater at the same rate as the rain recharging it, then we won’t run out. If we use the freshwater faster than it recharges, then we will. Intermediate renewable resources must be carefully managed to ensure they are not depleted.
Non-renewable Resources
The last category of natural resources are the non-renewables. These are resources that will not regenerate on human time-scales. Once they have been depleted they will no longer be available and no more will be made. The most common examples of non-renewable resources are fossil fuels, so-called because most were created by processes that take millions of years. Fossil fuels include crude oil, natural gas, coal and uranium. Other non-renewable resources include metals, lithium and rare-Earth elements (REE’s), but it’s important to remember that while we may eventually run out of mineable metals and REE’s, with careful waste management, these can be recovered through recycling. However, it is not the same for fossil fuels as using them for energy alters their chemistry so they are no longer useful.
Posted by: Venturit Super Admin
Disciplinary Content
Host: MSU Libraries

Intro to 360 Room @ DSL: Drop-in Session
The 360 Room at the DSL is your gateway to collaborative learning, teaching and shared experiences. Look at work created by other MSU faculty, staff and students and start thinking about how you can take that next step to better present and engage, here and remotely across a range of disciplines.
Navigating Context
Host: MSU Libraries

Birding with the MSU Libraries: Accessible Birding Event
In conjunction with One Grand Read, we are hosting a beginner birder's outing to get you outside to watch birds. This will be a stationary birding event (no walking). We will teach you how to use binoculars to spot birds, and how to identify birds once you've found one. Everyone is welcome! Meet up at the picnic tables by Beal Gardens along the sidewalk by the river to look for and identify birds together!
Navigating Context
EXPIRED



