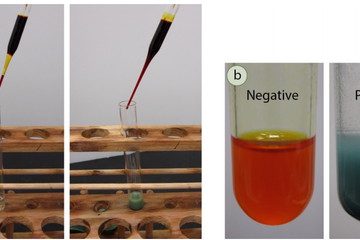
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color